Melliferous plants are natural source of nectar and pollen for honeybees and their pollen grains are present in honey. The melissopalynological study of Sabal yapa honey produced in Yucatan has shown that eighteen species contribute importantly to its honey composition. We use melliferous plants associated with Sabal yapa honey to test the potential of three barcodes (rbcL, ITS2, and trnH-psbA) in species identification to confirm the botanical origin and authentication of honey. Secondarily, we test the success rates of amplification and sequencing of each barcode. A total of 38 sequences were generated: 10 for rbcL, 13 for ITS2 and 15 for trnH-psbA. The success rate in PCR amplification was 94.73 % for trnH-psbA and 84.21 % for ITS2, while for rbcL was 52.63 %. Regarding sequencing success, 100 % of the products were amplified with rbcL. For trnH-psbA and ITS2, the sequencing success was 88.9 % and 87.5 %, respectively. ITS2 produced a species identification efficiency of 46.15 %, followed by rbcL (30 %). For trnH-psbA, the rate of species identification was 13.33 %. The sequences generated in this work will allow the construction of a reliable library of barcodes of melliferous plants, and with forward increasing will be possible to have a solid base for metabarcoding studies in the honey of the Yucatan Peninsula.
Keywords: honey, rbcL, ITS2, trnH-psbA.
As plantas melíferas são fonte natural de néctar e pólen para as abelhas, e seus grãos de pólen estão presentes na composição do mel. O estudo melissopalinológico do mel de Sabal yapa produzida em Yucatan mostrou que 18 espécies contribuem de forma importante para a composição do mel. Usamos plantas melíferas associadas ao mel de Sabal yapa para testar o potencial de três códigos de barras (rbcL, ITS2 e trnH-psbA) na identificação de espécies para confirmar a origem botânica e a autenticação do mel. Em segundo lugar, testamos as taxas de sucesso de amplificação e sequenciamento de cada código de barras. Foi generado um total de 38 sequências: 10 para rbcL, 13 para ITS2 e 15 para trnH-psbA. O sucesso da amplificação por PCR foi: trnH-psbA (94,73 %), ITS2 (84,21 %), rbcL (52,63 %). Com relação ao sucesso do sequenciamento, 100 % dos productos foram amplificados com rbcL (100 %). Para trnH-psbA e ITS2, o sucesso do sequenciamento foi de 88,9 % e 87,5 %, respectivamente. O ITS2 produziu uma eficiência de identificação de espécies de 46,15 % seguido por rbcL (30 %). A eficiência do trnH-psbA para identificar as espécies foi de 13.33 %. As sequências geradas neste trabalho permitirã construir uma biblioteca confiável de códigos de barras de plantas melíferas e, com o aumento futuro, será possível ter uma base sólida para estudos de metabarconding no mel da Península de Yucatán.
Palavras-chave: mel, rbcL, ITS2, trnH-psbA.
Introduction
DNA barcodes are small, standardized DNA fragments (500 to 800 base pairs bp) that are amplified and sequenced to identify organisms (Taberlet et al., 2012). This tool has helped to understand biological diversity and is currently applied to solve questions of systematics and phylogeny; as well as in the identification of parasites and vectors, the creation of forest and animal inventories, the detection of trafficking of endangered species, and the authentication of pharmaceutical and food products such as honey (Ajmal et al., 2014; Ferreira de Lima et al., 2018). Therefore, selecting universal DNA fragments as barcodes is a critical step for their implementation (Saravanan et al., 2019).
The Consortium for the Barcoding of Life (CBOL) has proposed rbcL as a core barcode in plants and several markers such as trnH-psbA or ITS2 as complementary barcodes to increase the species-level discrimination (Hollingsworth et al., 2009; Manivanan et al., 2018). The utility of these markers to corroborate honey authenticity has been evaluated by several authors (Bruni et al., 2015; Hawkins et al., 2015; Laha et al., 2017; Manivanan et al., 2018; Murthy et al., 2019; Prosser and Hebert 2017; Saravanan et al., 2019).
In tropical regions, where organism diversity is high and sampling difficult, barcode generation and implementation may be limited (Parmentier et al., 2013; Ferreira de Lima et al., 2018; Jones et al., 2021) as barcodes require reference sequences available on platforms such as GenBank or BOLD Systems for comparison and species identification (Hollingsworth et al., 2009; Jones et al., 2021). In Mexico, the generation of barcodes for melliferous plants is scarce (Hernández-Pineda, 2016). Only about 10,745 species including plants and animals have barcodes (103,190 records), of which 24.83 % correspond to vascular plants and 17 % to eudicotyledons (BOLD Systems, 2023a). Constructing reference libraries with reference sequences of well-verified specimens will allow correct identification by barcoding and more efficient analysis (Jones et al., 2021). For that reason, utilizing specimens available in herbaria is a key element in constructing solid databases (de Vere et al., 2012).
In the international markets where monofloral honey has a high value due to its organoleptic characteristics (Murthy et al., 2019) and honey may be subject to fraudulent practices (Prosser and Hebert 2017; Soares et al., 2017), DNA barcodes emerge as an innovative technique to authenticate honey and to determine its botanical origin (Soares et al., 2017). Since the Yucatan Peninsula is the most productive beekeeping region in Mexico (Servicio de Información Agroalimentaria y Pesquera, 2020) with 22 documented monofloral honey (Villanueva-Gutiérrez et al., 2009; Alfaro-Bates et al., 2010) and around 80 % the honey produced in this region is exported mainly to European countries (Cruz-Zamudio, 2017), the generation of DNA barcodes for melliferous plants of Yucatan is a critical step to identify potential barcodes to authenticate honey. We selected 18 species of plants associated with Sabal yapa monofloral honey to evaluate the success rates of amplification, sequencing, and identification of three barcodes (rbcL, ITS2, and trnH-psbA) and identify potential barcodes in melliferous plants.
Materials and Methods
Selection of samples
Eighteen species were selected according to the melissopalynological analysis of Sabal yapa honey from Tizimin, Yucatan (Durán-Escalante et al., 2023) (Table 1). Seven of these species were the major contributors of pollen to Sabal yapa honey and are also the major plant contributors to honey production in the Yucatan Peninsula (Alfaro-Bates et al., 2010). Four species provided pollen categorized as minor or important minor pollen, and seven species are part of the group that we consider can help to give certainty to the pollen grains that could only be identified by palynology at the botanical family level (i.e., Malvaceae, Asteraceae) in Sabal yapa honey. The plants were gathered around the apiaries of a local beekeeper where this honey is harvested. Lately, the dried specimens were identified by expert taxonomists and deposited in the herbarium of the Universidad Autónoma de Yucatán (UADY). The specimens are available online in the portal of the Red de Herbarios del Noroeste de México (2023).
Table 1. List of species and vouchers used for DNA barcode generation and Process ID generated in BOLD Systems platform for studied species.
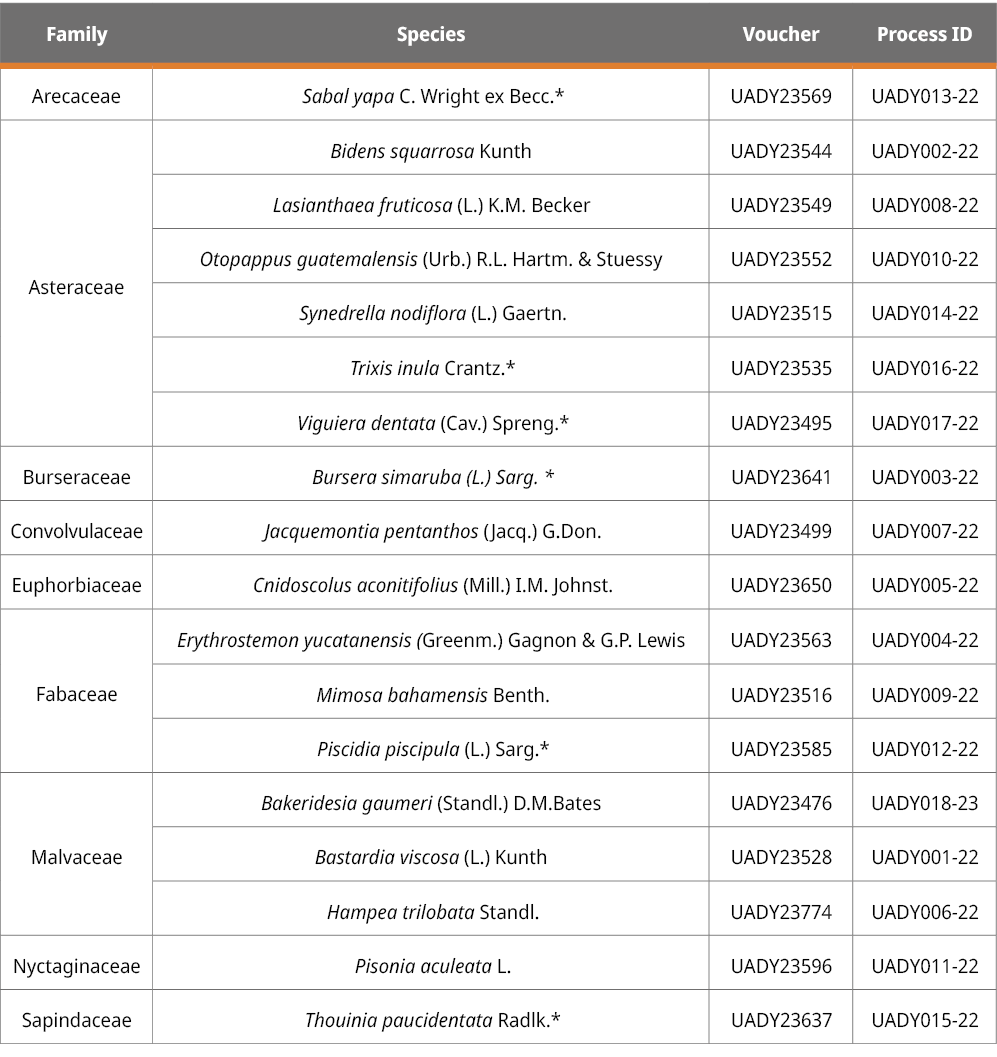
*Main species that contribute to honey production in Yucatan.
DNA isolation and Polymerase Chain Reaction (PCR)
Total genomic DNA extraction was performed from 20 mg of leaf tissue (freshly taken and transferred to the laboratory in a bag with silica gel at room temperature) using the DNeasy Plant mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. Yields and quality of DNA were verified by agarose gel electrophoresis (0.75 %) and spectrophotometer (Jenway 737501 Genova Nano Micro-spectrophotometer, United Kingdom). DNA barcodes were amplified by PCR using universal primers for rbcL (Kress and Erickson, 2007; Fazekas et al., 2008), ITS2 (Chiou et al., 2007) and trnH-psbA (Sang et al., 1997; Tate and Simpson, 2003). The primer sequences (5’ to 3’) were rbcLa_F (ATG TCA CCA CAA ACA GAG ACT AAA GC) and rbcLajf634 (GAA ACG GTC TCT CAA ACG CAT); ITS-S2 F (ATG CGA TAC TTG GTG TGA AT) and ITS2 R (GAC GCT TCT CCA GAC TAC AAT); trnH-psbA F (GTT ATG CAT GAA CGT AAT GCT C) and trnH-psbA R (CGC GCA TGG TGG ATT CAC AAT CC).
All PCR reactions were performed on a MultiGene TC020-24 thermal cycler (Labnet International, China) using a reaction mixture of a total volume of 25 or 50 ml containing 12.5 or 25 ml of GoTaq® Green Master Mix 2X, 1.5 ml forward and reverse primer each (10 mM), 0.5 ml of BSA (0.04 %), and template DNA (< 250 ng). The reaction volume was topped up to 25 or 50 ml using nuclease - free water. The PCR conditions for amplification of rbcL were initial denaturation at 94 ℃ 4 min, followed by 35 cycles of 94 ℃ 30 s, 53 ℃ 30 s, and 72 ℃ 60 s; extension at 72 ℃ 10 min, and hold 4 ℃. The PCR conditions for amplification of ITS2 were initial denaturation at 94 ℃ 3 min, followed by 35 cycles of 95 ℃ 30 s, 48 ℃ 30 s, and 72 ℃ 30 s; extension at 72 ℃ 7 min, and hold 4 ℃. Finally, the PCR conditions for amplification of trnH-psbA were initial denaturation at 95 ℃ 2 min and 30 s, followed by 35 cycles at 95 ℃ 30 s, 49 ℃ 30 s, 64 ℃ 60 s; extension at 72 ℃ 7 min, and hold 4 ℃. PCR products were visualized on 0.75 % agarose gels stained with SYBR Safe DNA under ultraviolet light. Amplified products were sent to Macrogen (Seoul, South Korea) for purifying and DNA sequencing using the same primers as in the amplification.
DNA sequence editing, nucleotide Basic Local Alignment Seach Tool (BLAST) and dendrogram construction
The assembly of the sequences was performed in Sequencher 4.1.4 (Genes Codes Corporation, 2002) removing low-quality segments at two ends of the sequences by manual editing. Efficiencies of the three markers for species identification were evaluated using the nucleotide Basic Local Alignment Seach Tool (BLAST), which employs a local alignment and searches for segments with the highest score (McGinnis and Madden, 2004; Newell et al., 2013) in the NCBI platform (National Center for Biotechnology Information, 2023). The default database selected is Nucleotide Collection (nr/nt), and highly similar sequences (megablast) as program selection. The query sequence was considered correctly assigned when a) the percentage of similarity corresponded to ≥ 99 % with the reference sequences in the database and b) the analyzed sequence matched the species name. When the percentage of identity was < 99 %, the identification was considered incomplete because of the absence of specific sequences in the GenBank (Aghayeva et al., 2021).
The sequences edited were aligned in MEGA 11 (Tamura et al., 2021) with MUSCLE (Edgar, 2004) for multiple alignment including sequences available on the GenBank platform for the genus or species (downloaded on September 22, 2022). A phenetic dendrogram was elaborated for each marker with the multiple alignment matrix to corroborate visually the BLAST results obtained. This strategy uses a global alignment where the alignment strain along the length of both sequences (Ignacimuthu, 2005) and allows the recognition of clusters of very similar sequences due to their high level of similarity (Peña, 2011). Trees were constructed by Neighbor-Joining (NJ) method (Saitou and Nei, 1987). The marker was considered an efficient barcode for species identification when the query sequence clustered into a group with the same species or genus sequences. Analyses were conducted in MEGA 11 (Tamura et al., 2021) using the Maximum Composite Likelihood method (Tamura et al., 2004) and a Bootstrap test (1000 replicates) (Felsenstein, 1985). Finally, all information related to the specimens (sequences, taxonomic description, voucher number assigned by the herbarium, collection date, and georeferencing) was uploaded to GenBank and BOLD Systems v4 platforms (BOLD Systems, 2023b) as part of the project “UADY DNA Barcoding of melliferous plants in the Yucatan Peninsula (Tizimin)”.
Results
The universality of primer sequences
DNA extracted from leaf tissue samples was amplified for the three target regions using a single pair of universal primers for each locus. A total of 38 sequences were obtained: 10 for rbcL, 13 for ITS2, and 15 for trnH-psbA. The rbcL fragment showed an amplification success rate of 55.55 %, ITS2 83.33 % and trnH-psbA 94.44 %. Of the nine botanical families studied, rbcL and ITS2 amplified for all except Convolvulaceae. The trnH-psbA fragment amplified for all families and species except for Bakeridesia gaumeri (AB12), a member of the Malvaceae family.
Regarding DNA sequencing, the success rate of sequencing was 100 % for rbcL, followed by trnH-psbA (88.9 %) and ITS2 (87.5 %). In all cases, amplification products of the expected size were obtained. The average length of the rbcL sequences was 631 bases, ITS2 of 473 bases, and trnH-psbA of 509 bases. The latter fragment exhibited a variation in the length of the sequences between species. Although Sabal yapa and Cnidoscolus aconitifolius amplified for ITS2, overlapping peaks were found in sequencing results and could not be used for further analysis. The same occurred with Mimosa bahamensis with the trnH-psbA fragment, where the electropherograms were unclear.
Identification efficiency
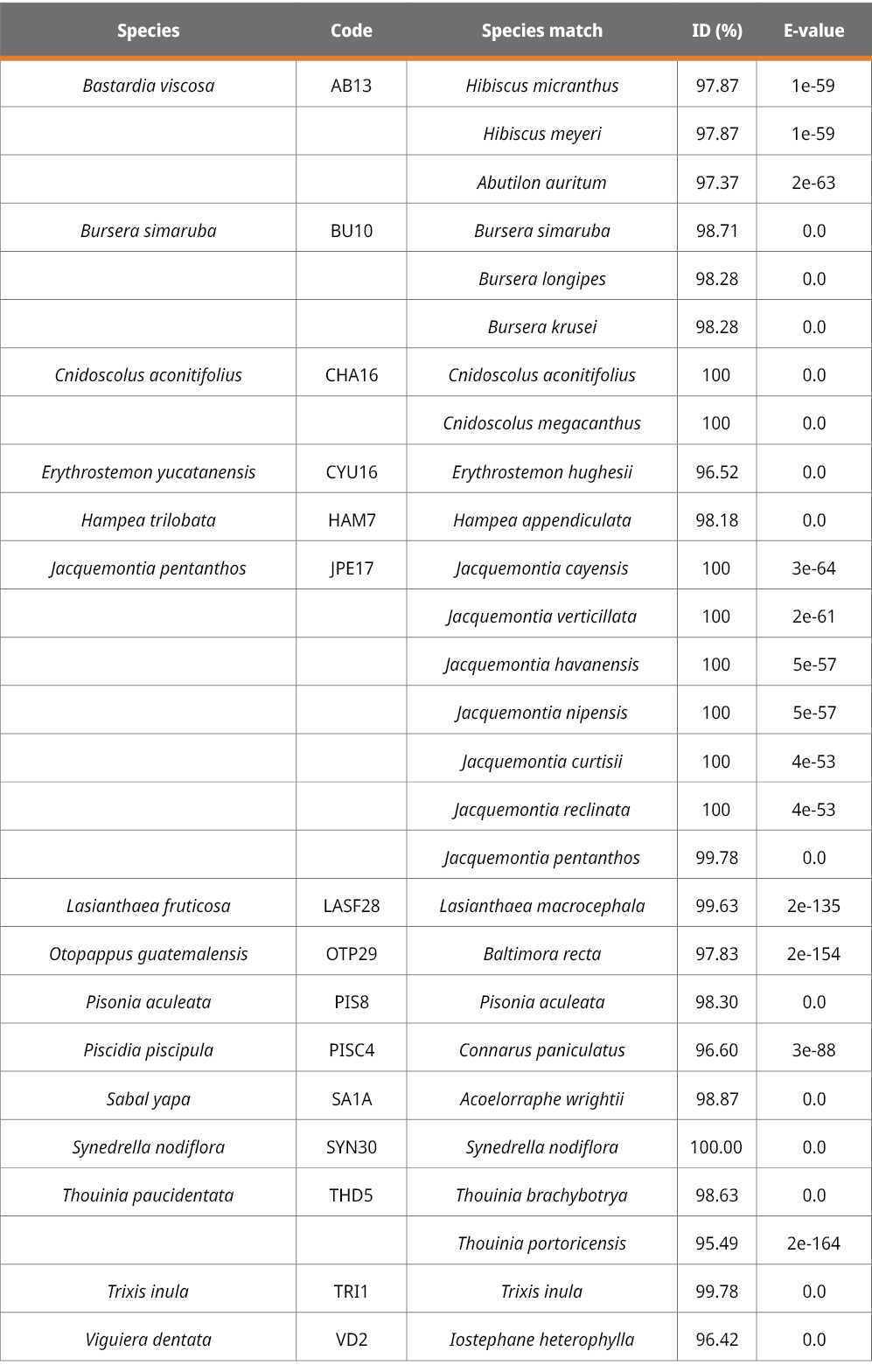
The rbcL, ITS2 and trnH-psbA markers are suitable for plant identification but at different levels. Using the BLAST method to identify species with given samples, the success of species identification with ITS2 was 46.15 %, followed by rbcL (30 %) and trnH-psbA (13.33 %). From 10 rbcL sequences, three could be assigned to the species level, three to the genus level, and four to the family level (Table 2). Six out of the 13 ITS2 sequences could be correctly assigned to the species level, five at the genus level, and two at the family level (Table 3). Finally, from the 15 trnH-psbA sequences, only two could be correctly assigned to the species level. Of the rest, eight were assigned to the genus level and five to the family level (Table 4).
Table 2. BLAST results for the rbcL region in plant samples collected in this study.
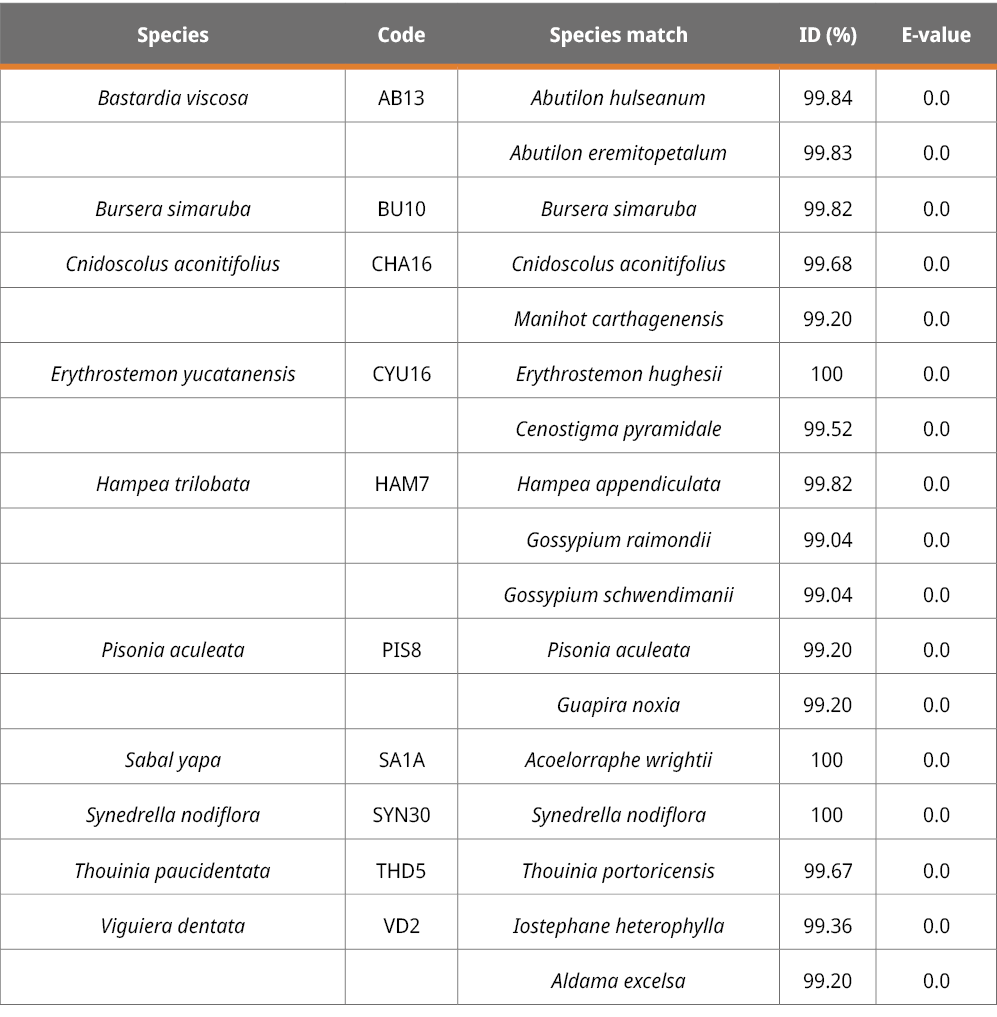
Table 3. ITS2 region in plant samples collected in this study.
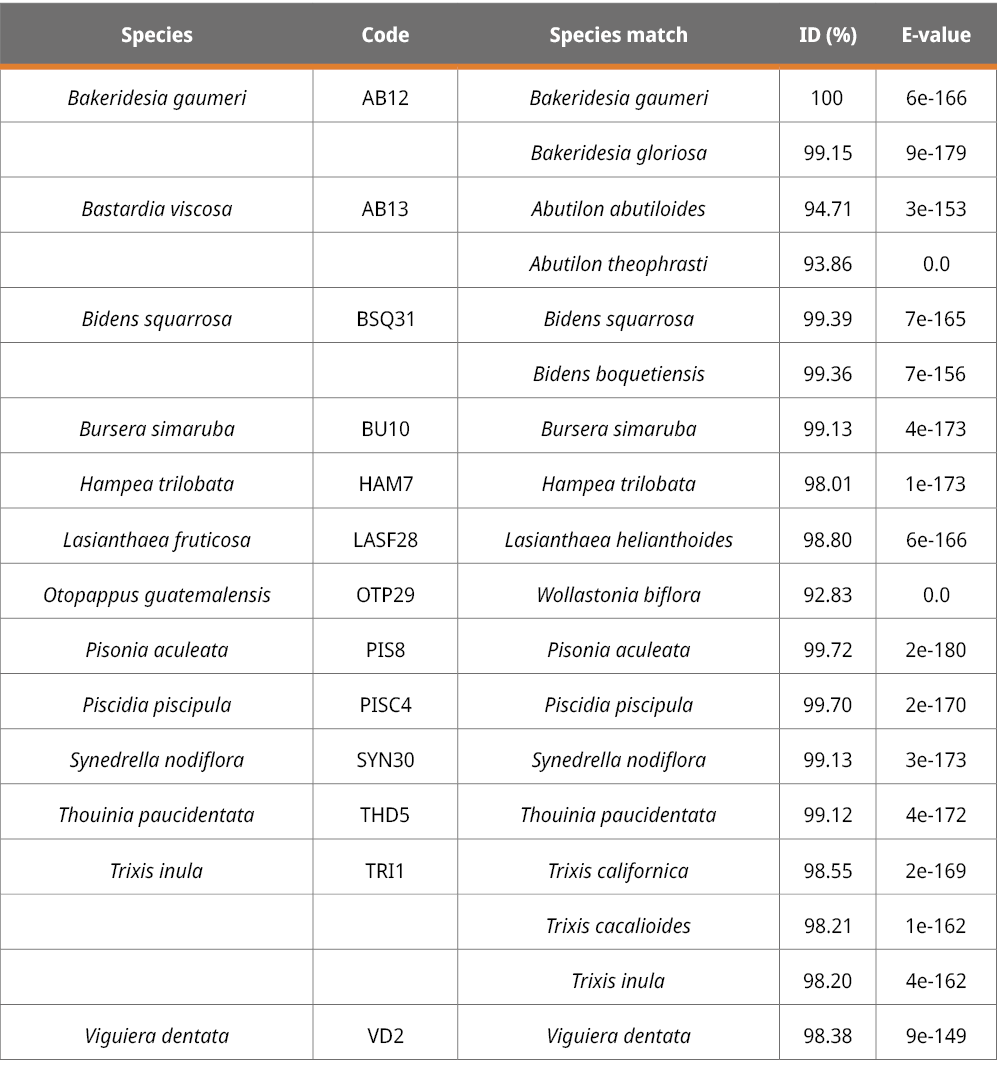
Table 4. BLAST results for the trnH-psbA region in plant samples collected in this study.
When distance methods were used for species identification, Neighbor-Joining trees showed our sequences cluster correctly with GenBank sequences for the same species or genus (in a few cases with the same family members). The rbcL sequences were correctly grouped by genus except for Bastardia viscosa, which was grouped with other Malvaceae species because it does not have reference sequences in GenBank (Figure 1). The tree size for ITS2 is larger than for the other two markers due to the availability of reference sequences for the organisms studied (Figure 2). Although the species identification efficiency of trnH-psbA using BLAST was low, species formed well-defined groups in trees. Since Otoppapus guatemalensis has no reference sequences for trnH-psbA in GenBank, the sequence clustered with other species of Asteraceae but on a different branch within the tree (Figure 3).
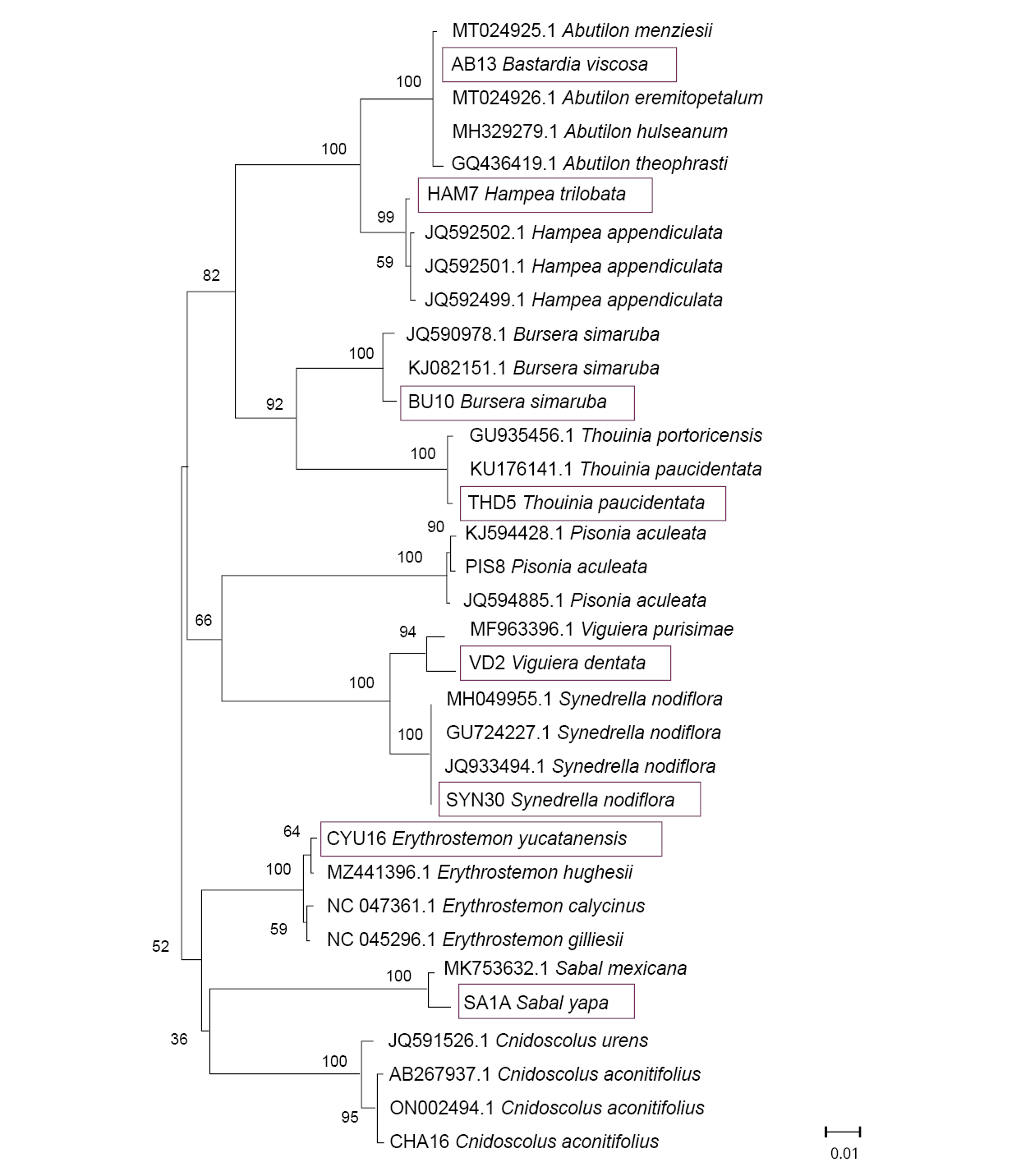
Figure 1. Neighbor-Joining (NJ) tree, based on rbcL sequences. Above the branches, the Bootstrap value is shown. The blue rectangles indicate the location of our samples in the corresponding clusters. Matrix variability was 23.01 %.
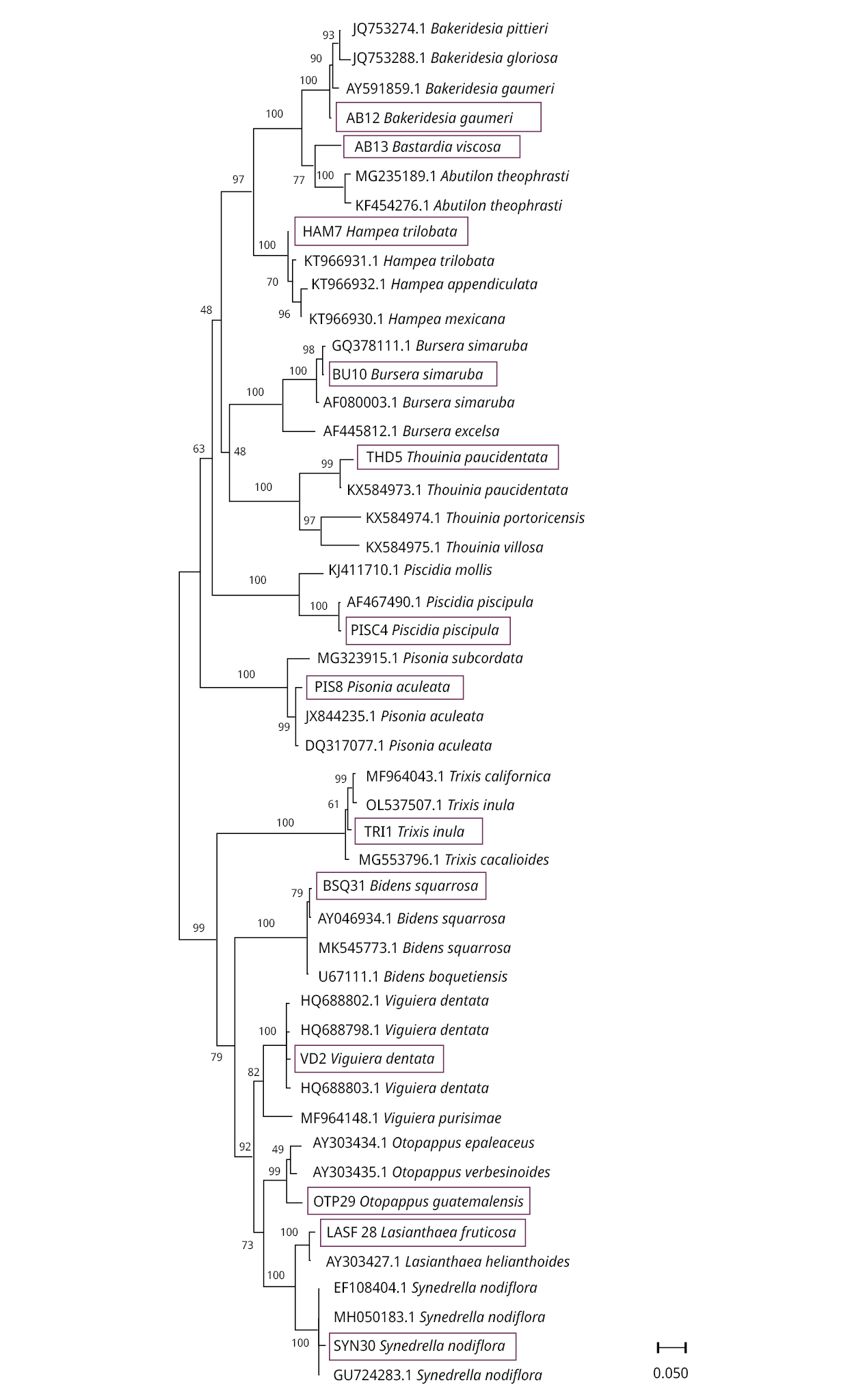
Figure 2. Neighbor-Joining (NJ) tree, based on ITS2 sequences. Above the branches, the Bootstrap value is shown. The blue rectangles indicate the location of our samples in the corresponding clusters. Matrix variability was 64.91 %.
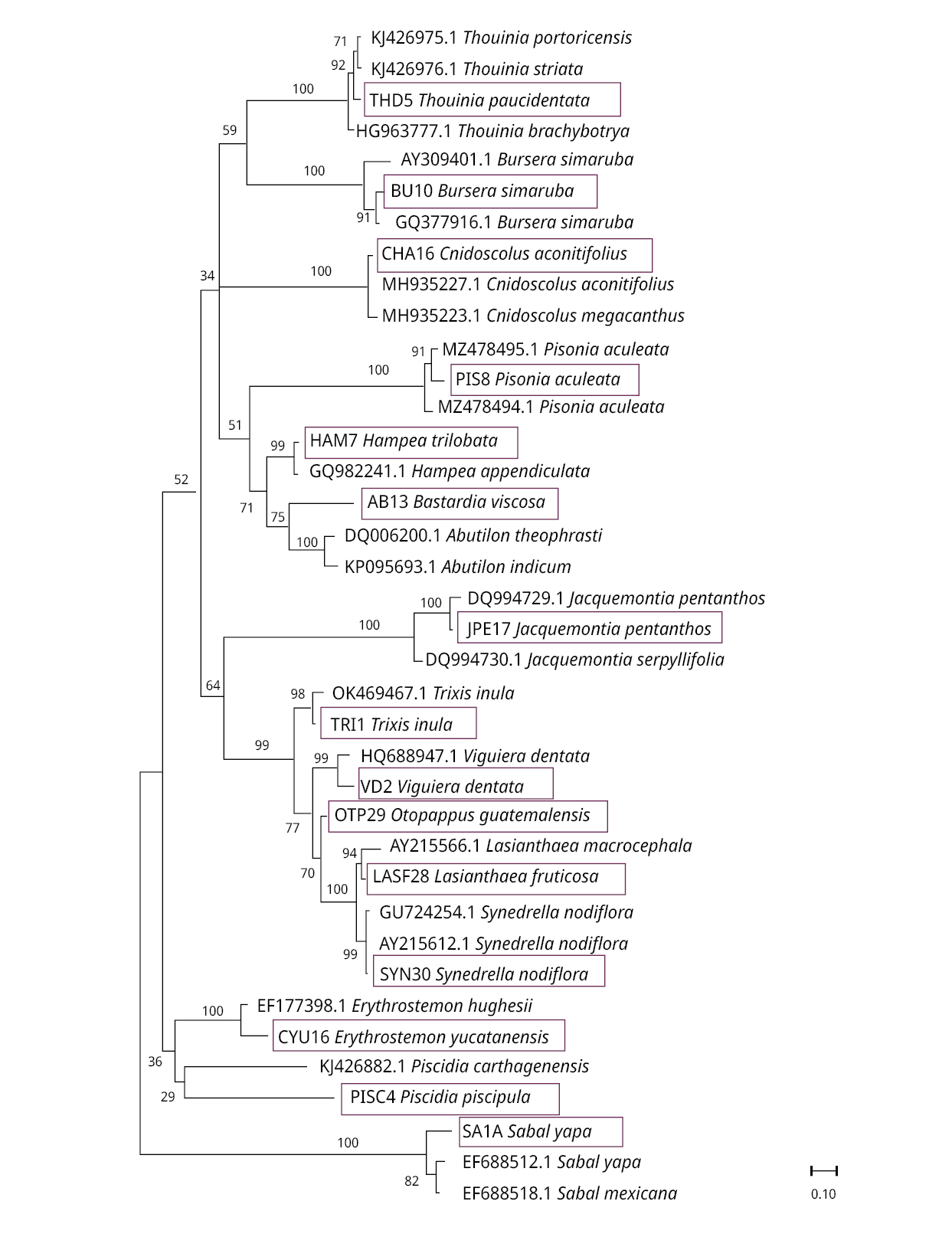
Figure 3. Neighbor-Joining (NJ) tree, based on trnH-psbA sequences. Above the branches, the Bootstrap value is shown. The blue rectangles indicate the location of our samples in the corresponding clusters. Matrix variability was 75 %.
Discussion
Differences between amplification and sequencing success were observed between the three regions using a single pair of universal primers. Four plant species belonging to the Asteraceae family (Bidens squarrosa, Lasianthaea fruticosa, Otopappus guatemalensis, and Trixis inula) did not amplify using the universal rbcL (rbcLa-F/rbcLaif634) primers. This has also been observed in other Asteraceae species using rbcLa/rbcLa R and rbcL1F/rbcL-724R primers (Bafeel et al. 2011) probably due to a primer mismatch at the 3’ end of the primer sequence (Bru et al., 2008). For this reason, the combination of several reverse primers is required to amplify successfully in different groups of plants (de Vere et al., 2012). In contrast to rbcL, amplification rates for ITS2 and trnH-psbA were higher and agree with those obtained by several authors where ITS2 amplifies between 84.39 % and 93.8 % (Gong et al., 2018; Chen et al., 2010, Gao et al., 2010) and trnH-psbA 72.2 % and 92.8 % (Chen et al., 2010; Gao et al., 2010; Kang et al., 2017).
Hollingsworth et al. (2009) have reported obtaining high-quality bidirectional rbcL sequences like those we got in our study (100 %). In some species, secondary metabolites can interfere with the DNA extraction causing PCR amplification and sequencing failures (Bafeel et al., 2011; Kang et al., 2017). That was probably the case for Mimosa bahamensis (trnH-psbA), Cnidoscolus aconitifolius (ITS2), and Sabal yapa (ITS2), where purity indicators A260/280 and A260/230 were lower than 1.8.
The discriminatory capacity of each marker relies on nucleotide substitution rates and the availability of sequences in public databases (Aghayeva et al., 2021; Jones et al., 2021). On one hand, it is essential for a marker to exhibit species-level differentiation at sequence level, to discriminate biological entities effectively (Casiraghi et al., 2010). Moreover, the presence or absence of sequences from specific interest groups in the reference databases can significantly impact the efficiency of barcodes (Rey Bentos and Capdevielle Sosa, 2020; Taberlet et al., 2007). Therefore, the likelihood of accurately assigning a query sequence also depends on the completeness of sequences available for the target taxa in public databases (BOLD Systems, 2023c).
The rbcL marker has a conservative rate of nucleotide substitution, which makes this marker very useful for plant identification at the genus or family level (Martínez, 1997). Despite its limited ability to identify closely related species (Bruni et al., 2015; Saravanan et al., 2019), it is chosen as a core barcode to maintain a certain degree of compatibility with the genetic repositories as established by the CBOL (Galimberti et al., 2014) and has many reference sequences for many species. The internal transcribed spacer 2 (ITS2) is a highly variable region (Newmaster et al., 2013; Duan et al., 2019), suitable for genera and species identification because, in general, the spacers evolve faster than coding regions (Poczai and Hyvönen, 2010). This barcode has many sequences in public databases and is the locus more used in pollen DNA barcoding studies (Bell et al., 2016, Milla et al., 2022). In this study, ITS2 had the highest species identification efficiency (46.15 %) and it is consistent with the obtained from other authors in diverse barcoding plant identification projects (Chen et al., 2010; Gao et al., 2010; Liu et al.,2019; Newmaster et al., 2013).
Some authors have highlighted that trnH-psbA can correctly identify plants at the genus level (between 90 % and 98 %) better than rbcL (Loera-Sánchez et al., 2020; Parmentier et al., 2013) as we observed in our study, where trnH-psbA correctly assigned eight sequences at the genus level against three rbcL sequences. At the species level, trnH-psbA by itself (Parmentier et al., 2013; Loera-Sánchez et al., 2020) or as a compliment to ITS2 (Liu et al., 2019; Pang et al., 2012) or rbcL (Galimberti et al., 2014; Parmentier et al., 2013) shows high efficiency in the identification of plants but in our case, we observed the opposite. For example, trnH-psbA could not differentiate correctly between Cnidoscolus aconitifolius and C. megacanthus and Jacquemontia pentanthos vs. other six species. Second, we obtained only 13.33 % correct assignment to species level with this marker. This is partly because 16 of the18 species, have few or no reference sequences for this region in public databases to allow comparison. The scarce availability of this region in the databases is associated with problems obtaining bidirectional sequences due to high mononucleotide repeats (Bruni et al., 2015; Hollingsworth et al., 2009). Despite its limitations for species identification in our study, it seems to be a suitable genus-level marker used as DNA barcode to characterize honey from a limited geographic area with well-known flora (Bruni et al., 2015) such as Sabal yapa honey produced in Tizimin, Mexico.
BLAST and dendrograms are different strategies to explore and evaluate the usefulness of barcodes for species identification (Bolson et al., 2015). In a heuristic search, BLAST attempts to find the best overall results after comparing a query sequence with an entire public nucleotide database (McGinnis and Madden, 2004). Nevertheless, BLAST has a bias for hits that contain smaller or no gaps over hits with long gaps, so alignments can fail to identify species correctly (Dereeper et al., 2010). BLAST analyses with ITS2 failed for example to correctly assign Trixis inula with its reference sequence (98.20 %) and showed a high percentage of similarity with T. californica (98.55 %) and T. calicoides (98.21 %) but in the dendrograms, the query sequence clustered correctly with the reference species, demonstrating that distance methods are adequate for corroborating BLAST results.
Lastly, we underscore the significance of producing accurately identified plant barcodes, supported by herbarium vouchers for validation purposes. In this study, we identified a reference sequence labeled in GenBank as Asteraceae sp. (Accession HG964019.1), which exhibited a 100 % match with the query sequence TRI1 (Trixis inula) for trnH-psbA region.
Conclusions
In this work, we generated barcodes for the most important melliferous plants associated with Sabal yapa honey production of Eastern Yucatan with well-verified specimens. None of the three markers evaluated could independently identify all the studied species successfully. Nonetheless, the results from this preliminary study suggest that the ITS2 marker demonstrated favorable amplification and sequencing rates using a single primer pair, leading to more accurate species identification. This increased accuracy is attributed, in part, to the availability of reference sequences in GenBank. This first approach suggests the potential of ITS2 as a candidate barcode for honey flora. Furthermore, we recommend continued efforts in generating the trnH-psbA barcode for melliferous species to assess its utility as a honey barcode in the Yucatan Peninsula. Additionally, it is valuable to use other commercially significant monofloral honey as models to replicate this study, broadening the coverage of melliferous species. Generating reference sequences from well-verified specimens will provide a comprehensive representation of these species in public databases, and these sequences can be employed with utmost confidence for future analyses (e.g., metabarcoding, food authentication) specifically targeting the species of interest.
Acknowledgments
We thank Javier Cuxim for allowing us to collect specimens in his apiaries, Alexander Suárez-Mariño for his help during the field collections, and finally Geovani Palma Pech for processing specimens.
First author Kelly Durán Escalante was granted scholarship number 2020-000013-01 NACF-00460 by the Consejo Nacional de Ciencia y Tecnología (CONACYT) to pursue her doctoral studies.
References
Aghayeva, P.; Cozzolino, S.; Cafasso, D.; Ali-zade, V.; Fineschi, S. and Aghayeva, D., 2021. DNA barcoding of native Caucasus herbal plants: potentials and limitations in complex groups and implications for phylogepgraphic patterns. In: Biodiversity Data Journal, 9, e61333. DOI: https://doi.org/10.3897/BDJ.9.e61333
Alfaro-Bates, R.; González Acereto, J.; Ortiz Díaz, J.; Viera Castro, F.; Burgos Pérez, A.; Martínez Hernández, E. and Ramírez Arriaga, E., 2010. Caracterización palinológica de las mieles de la Península de Yucatán. Mérida, México: UADY-CONABIO. ISBN: 978-607-7573-42-5.
Ajmal, A.M.; Gyulai, G.; Hidvégi, N.; Kerti, B.; al Hemaid, F.M.A.; Pandey, A.K. and Lee, J., 2014. The changing epitome of species identification – DNA barcoding. In: Saudi Journal of Biological Sciences, 21(3), pp. 204-231. DOI: https://doi.org/https://doi.org/10.1016/j.sjbs.2014.03.003
Bafeel, S.O.; Arif, I.A.; Bakir, M.A.; Khan, H.A.; Al Farhan, A.H.; Al Homaidan, A.A.; Ahamed, A. and Thomas, J., 2011. Comparative evaluation of PCR success with universal primers of maturase K (matK) and ribulose-1, 5-bisphosphate carboxylase oxygenase large subunit (rbcL) for barcoding of some arid plants. In: Plant Omics Journal, 4(4), pp.195-198.
Bell, K.L.; de Vere, N.; Keller, A.; Richardson, R.T.; Gous, A.; Burgess, K.S. and Brosi, B.J., 2016. Pollen DNA barcoding: current applications and future prospects. In: Genome 59(9), pp.629-640. DOI: https://doi.org/10.1139/gen-2015-0200
Barcode of Life Data Systems, BOLD Systems, 2023a. Barcode of Life Data Systems v4 [Online]. Ontario: BOLD Systems. [Accessed: May 22 of 2023]. Available at: https://www.boldsystems.org/index.php
Barcode of Life Data System v4, BOLD Systems, 2023b. UADY DNA Barcoding of melliferous plants in the Yucatan Peninsula (Tizimin) [Online]. Ontario: BOLD Systems. [Accessed: June 22 of 2023]. Available at: https://www.boldsystems.org/
Barcode of Life Data Systems, BOLD Systems, 2023c. Barcode of life data systems handbook. A web-based bioinformatics platform supporting the DNA barcoding of animal, plant, and fungal species [Online]. Ontario: BOLD Systems. [Accessed: May 22 of 2023]. Available at: https://bit.ly/44PvwoL
Bolson, M.; Smidt, Ed.; Brotto, M.L. and Silva-Pereira, V., 2015. ITS and trnH-psbA as efficient DNA barcodes to identify threatened commercial woody angiosperms from Southern Brazilian Atlantic rainforests. In: PLOS ONE, 10(12), e0143049. DOI: https://doi.org/10.1371/journal.pone.0143049
Bru, D.; Laurent, F.M. and Philippot, L., 2008. Quantification of the detrimental effect of a single primer-template mismatch by real-time PCR using the 16s rRNA gene as an example. In: Applied and Environmental Microbiology, 74(5), pp. 1660-1663. DOI: https://doi.org/10.1128/AEM.02403-07
Bruni, I.; Galimberti, A.; Caridi, L.; Scaccabarozzi, D.; de Mattia, F.; Casiraghi, M. and Labra, M., 2015. A DNA barcoding approach to identify plant species in multiflower honey. In: Food Chemistry, 170, pp. 308-315. DOI: https://doi.org/10.1016/j.foodchem.2014.08.060
Casiraghi, M.; Labra, M.; Ferrri, E.; Galimberti, A. and De Mattia, F., 2010. DNA barcoding: a six-question tour to improve user’s awareness about the method. In: Briefing in Bioinformatics, 2(4), pp. 440-453. DOI: https://doi.org/10.1093/bib/bbq003
Chen, S.L.; Yao, H.; Han, J.P.; Chang, L.; Song, J.Y.; Shi, L.; Zhu, Y.; Ma, X.; Gao, T.; Pang, X.; Luo, K.; Li, Y.; Li, X.; Jia, X.C.; Lin, Y. and Leon, C., 2010. Validation of the ITS2 region as a novel DNA barcode for identifying medicinal plant species. In: PLOS ONE, 5, e8613. DOI: https://doi.org/10.1371/journal.pone.0008613
Chiou, S.J.; Yen, J.H.; Fang, C.L.; Chen, H.L. and Lin, T.Y., 2007. Authentication of medicinal herbs using PCR-amplified ITS2 with specific primers. In: Planta Medica, 73 (13), pp.1421-1426. DOI: https://doi.org/10.1055/s-2007-990227
Cruz-Zamudio, A., 2017. Producción de miel convencional y orgánica en la Península de Yucatán. Sherbrooke: El Colegio de la Frontera Sur – Université de Sherbrooke. (Tesis de Maestría).
de Vere, N.; Rich, T.C.G.; Ford, C.R.; Trinder, S.A.; Long, C.; Moore, C.W.; Satterthwaite, D.; Davies, H.; Allainguillaume, J.; Ronca, S.; Tatarinova, T.; Garbett, H.; Walker, K. and Wilkinson, M.J., 2012. DNA Barcoding the Native Flowering Plants and Conifers of Wales. In: PLOS ONE, 7(6), e37945. DOI: https://doi.org/10.1371/journal.pone.0037945
Dereeper, A.; Audic, S.; Claverie, J.M. and Blanc, G., 2010. LAST-EXPLORER helps you building datasets for phylogenetic analysis. In: BMC Evolutionary Biology, 10(8). DOI: https://doi.org/10.1186/1471-2148-10-8
Duan, H.; Wang, W.; Zeng, Y.; Guo, M. and Zhou, Y., 2019. The screening and identification of DNA barcodes sequences for Rehmannia. In: Scientific Reports, 9, 17295. DOI: https://doi.org/10.1038/s41598-019-53752-8
Durán-Escalante, K.; Ortiz-Díaz, J.J.; Pinzón-Esquivel, J.P.; Gálvez-Mariscal, M.A. and Alfaro-Bates, R.G., 2023. Palynological characterization of palm honey (Sabal yapa C. Wright ex Becc.) of Yucatan, Mexico. In: Grana 62(2), pp. 133-145. DOI: https://doi.org/10.1080/00173134.2023.2178264
Edgar, R.C., 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. In: Nucleic Acids Research, 32(5), pp. 1792-1797. DOI: https://doi.org/10.1093/nar/gkh340
Fazekas, A.J.; Burgess, K.S.; Kesanakurti, P.R.; Graham, S.W.; Newmaster, S.G.; Husband, B.C.; Percy, D.M.; Hajibabaei, M. and Barrett, S.C., 2008. Multiple multilocus DNA barcodes from the plastid genome discriminate plant species equally well. In: PLOS ONE, 3(7), e2802. DOI: https://doi.org/10.1371/journal.pone.0002802
Felsenstein, J., 1985. Confidence limits on phylogenies: An approach using the bootstrap. In: Evolution, 39(4), pp. 783-791. DOI: https://doi.org/10.1111/j.1558-5646.1985.tb00420.x
Ferreira de Lima, R.A.; de Oliveira, A.A.; Dalla-Colleta, G.; Bevilcqua-Flores, T.; Gayoso-Coelho, R.L., et al., 2018. Can plant DNA barcoding be implemented in species-rich tropical regions? A perspective from São Paulo State, Brazil. In: Genetics and Molecular Biology, 41(3), pp. 661-670. DOI: https://doi.org/10.1590/1678-4685-GMB-2017-0282
Galimberti, A.; De Mattia, F.; Bruni, I., Scaccabarozzi, A.; Sandionigi, A.; Barbuto, M.; Casiraghi, M. and Labra, M., 2014. A DNA barcoding approach to characterize pollen collected by honeybees. In: PLOS ONE, 9(10), e109363. DOI: https://doi.org/10.1371/journal.pone.0109363
Gao, T.; Yao, H.; Song, JY.; Zhu, YJ.; Liu, C. and Chen, SL., 2010. Evaluating the feasibility of using candidate DNA barcodes in discriminating species of the large Asteraceae family. In: BMC Evolutionary Biology, 10, 324. DOI: https://doi.org/10.1186/1471-2148-10-324
Genes Codes Corporation, 2002. Sequencher. Vers. 4.1.4. Ann Arbor: Genes Codes Corporation.
Gong, L.; Qiu X.H.; Huang, J.; Xu, W.; Bai, J.Q.; Zhang, J.; Su, H.; Xu, C.M. and Huang, Z.H., 2018. Constructing a DNA barcode reference library for the southern herbs in China: a resource for authentication of southern Chinese medicine. In: PLOS ONE, 13(7), e0201240. DOI: https://doi.org/10.1371/journal.pone.0201240
Hawkins, J.; de Vere, N.; Griffith, A.; Ford, C.R.; Allainguillaume, J.; Hegarty, M.J.; Baillie, L. and Adams-Groom, B., 2015. Using DNA metabarcoding to identify the floral composition of honey: a new tool for investigating honey bee foraging preferences. In: PLOS ONE, 10(8), e0134735. DOI: https://doi.org/10.1371/journal.pone.0134735
Hernández-Pineda, J.A., 2016. Códigos de barras biológicos en la identificación de plantas de importancia melífera. México: Universidad Nacional Autónoma de México. (Tesis de Maestría).
Hollingsworth, P.M.; Forrest, L.L.; Spouge, J.L.; Hajibabaei, M.; Ratnasingham, S.; van der Bank, M.; Chase, M.W.; Cowan, R.S.; Erickson, D.L.; Fazekas, A.J.; Graham, S.W.; James, K.E.; Kim, K.J.; Kress, W.J.; Schneider, H.; van AlphenStahl, J.; Barrett, S.C.H.; van den Berg, C.; Bogarin, D. …and Little, D.P., 2009. A DNA barcode for land plants. In: Proceedings of the National Academy of Sciences, 106(31), pp. 12794-12797. DOI: https://doi.org/10.1073/pnas.0905845106
Ignacimuthu, S., 2005. Basic bioinformatics. Harrow: Alpha Science International. ISBN: 1842652311.
Jones, L.; Twyford, A.D.; Ford, C.R.; Rich, T.C.G.; Davies, H.; Forrest, L.L.; Hart, M.L.; McHaffie, H.; Brown, M.R.; Hollingsworth, P.M. and Vere, N., 2021. Barcode UK: A complete DNA barcoding resource for the flowering plants and conifers of the United Kingdom. In: Molecular Ecology Resources, 21(6), pp. 2050-2062. DOI: https://doi.org/10.1111/1755-0998.13388
Kang, Y.; Deng, Z.; Zang, R. and Long, W., 2017. DNA barcoding analysis and phylogenetic relationships of tree species in tropical cloud forests. In: Scientific Reports, 7,12564. DOI: https://doi.org/10.1038/s41598-017-13057-0
Kress, W.J. and Erickson, D.L., 2007. A two-locus global DNA barcode for land plants: the coding rbcL gene complements the non-coding trnH-psbA spacer region. In: PLOS ONE, 2(6), e508. DOI: https://doi.org/10.1371/journal.pone.0000508
Laha, R.C.; de Mandal, S.; Ralte, L.; Ralte, L.; Kumar, N.S.; Gurusubramanian, G.; Satishkumar, R.; Mugasimangalam, R. and Kuravadi, N.A., 2017. Meta-barcoding in combination with palynological inference is a potent diagnostic marker for honey floral composition. In: AMB Express, 7,132. DOI: https://doi.org/10.1186/s13568-017-0429-7
Liu, M.; Li, X.W.; Liao, B.S.; Luo, L. and Ren, Y.Y., 2019. Species identification of poisonous medicinal plant using DNA barcoding. In: Chinese Journal of Natural Medicines, 17(8), pp. 0585-0590. DOI: https://doi.org/10.1016/S1875-5364(19)30060-3
Loera-Sánchez, M.; Studer, B. and Kölliker, R., 2020. DNA barcode trnHpsbA is a promising candidate for efficient identification of forage legumes and grasses. In: BMC Research Notes, 13(35). DOI: https://doi.org/10.1186/s13104-020-4897-5
Manivanan, P.; Rajagopalan, S.M. and Subbarayalu, M.S., 2018. Studies on authentication of true source of honey using pollen DNA barcoding. In: Journal of Entomology and Zoology Studies, 6(3), pp. 255–261.
Martínez, M., 1997. Sistemática molecular: comparación entre diferentes métodos y sus aplicaciones. In: Boletín de la Sociedad Botánica de México, 60, pp. 123-136. DOI: https://doi.org/10.17129/botsci.1525
McGinnis, S. and Madden, T.L., 2004. BLAST: at the core of a powerful and diverse set of sequence analysis tools. In: Nucleic Acids Research, 32(Suppl. 2). DOI: https://doi.org/10.1093/nar/gkh435
Milla, L.; Schmidt-Lebuhn, A.; Bovill, J. and Encinas-Viso, F., 2022. Monitoring of honey bee floral resources with pollen DNA metabarcoding as a complementary tool to vegetation surveys. In: Ecological Solutions and Evidence, 3, e12120. DOI: https://doi.org/10.1002/2688-8319.12120
Murthy, M.; Khandayataray, P.; Ralte, L.; Laha, R. and Samal, D., 2019. Identification of plant species in multiflower honey by ribulose-bisphosphate carboxylase gene (rbcL) coding region as barcode marker, Mizoram, Northeast India: An Indo: Burma hotspot region. In: Journal of Entomology and Zoology Studies, 7(3), pp.1475-1483.
National Center for Biotechnology Information, NCBI, 2023. Basic Local Alignment Search Tool [Online]. Maryland: NCBI. [Accessed: June 22 of 2023]. Available at: https://blast.ncbi.nlm.nih.gov/Blast.cgi
Newell, P.D.; Fricker, A.D.; Roco, C.A.; Chandrangsu, P. and Merkel, S.M., 2013. A small-group activity introducing the use and interpretation of BLAST. In: Journal of Microbiology & Biology Education, 14(2), pp. 238-243. DOI: https://doi.org/10.1128/jmbe.v14i2.637
Newmaster, S.G.; Grguric, M.; Shanmughanandhan, D. Ramalingam, S. and Ragupathy, S., 2013. DNA barcoding detects contamination and substitution in North American herbal products. In: BMC Medicine, 11, 222. DOI: https://doi.org/10.1186/1741-7015-11-222
Pang, X.; Liu, C.; Shi, L.; Liu, R.; Liang, D.; Li, H.; Cherny, S.S. and Chen, S., 2012. Utility of the trnH–psbA intergenic spacer region and its combinations as plant DNA barcodes: a meta-analysis. In: PLOS ONE, 7(11), e48833. DOI: https://doi.org/10.1371/journal.pone.0048833
Parmentier, I.; Duminil, J.; Kuzmina, M.; Philippe, M.; Thomas, DW.; Kenfack, D.; Chuyong, G.B.; Cruaud, C. and Hardy, O.J., 2013. How effective are DNA barcodes in the identification of African rainforest trees? In: PLOS ONE, 8(4), e54921. DOI: https://doi.org/10.1371/journal.pone.0054921
Peña, C., 2011. Métodos de inferencia filogenética. In: Revista Peruana de Biología, 18(2), pp. 265-267. DOI: https://doi.org/10.15381/rpb.v18i2.243
Poczai, P. and Hyvönen, J., 2010. Nuclear ribosomal spacer regions in plant phylogenetics: problems and prospects. In: Molecular Biology Reports, 37(4), pp. 1897-1912. DOI: >https://doi.org/10.1007/s11033-009-9630-3
Prosser, S.W.J. and Hebert, P.D.N., 2017. Rapid identification of the botanical and entomological sources of honey using DNA metabarcoding. In: Food Chemistry, 214, pp.183-191. DOI: https://doi.org/10.1016/j.foodchem.2016.07.077
Red de Herbarios del Noroeste de México, 2023. Colección Herbario Alfredo Barrera Marín (UADY-UADY) [Online]. México: Red de Herbarios del Noroeste de México. [Accesed: June 22 of 2023]. Available at: https://herbanwmex.net/portal/index.php
Rey Bentos, F. and Capdevielle Sosa, F.M., 2020. Aplicación del código de barras de ADN (DNA barcoding) para la identificación de especies vegetales de interés industrial. In: INNOTEC, 20, pp.117-138. DOI: https://doi.org/10.26461/20.06
Saitou, N. and Nei, M., 1987. The neighbor-joining method: A new method for reconstructing phylogenetic trees. In: Molecular Biology and Evolution, 4(4), pp. 406-425. DOI: https://doi.org/10.1093/oxfordjournals.molbev.a040454
Sang, T.; Crawford, D.J. and Stuessy, T.F., 1997. Chloroplast DNA phylogeny, reticulate evolution, and biogeography of Paeonia (Paeoniaceae). In: American Journal of Botany, 84, 84(8), pp.1120-1136. DOI: https://doi.org/10.2307/2446155
Saravanan, M.; Mohanapriya, G.; Laha, R. and Sathishkumar, R., 2019. DNA barcoding detects floral origin of Indian honey samples. In: Genome, 62(5), pp. 341-348. DOI: https://doi.org/10.1139/gen-2018-0058
Servicio de Información Agroalimentaria y Pesquera., 2023. Producción estatal de miel durante el año 2020 en México [Online]. Servicio de Información Agroalimentaria y Pesquera. [Accessed: June 12 of 2023] Available at: bit.ly/3ZbYMoF
Soares, S.; Amaral, J.S.; Oliveira, M.B.P.P. and Mafra, I., 2017. A Comprehensive review on the main honey authentication issues: production and origin. In: Comprehensive Reviews in Food Science and Food Safety, 16(5), pp. 1072-1100. DOI: https://doi.org/https://doi.org/10.1111/1541-4337.12278
Taberlet, P.; Coissac, E.; Pompanon, F.; Gielly, L. Miquel, C.; Valentini, A.; Vermat, T.; Corthier, G.; Brochmann, C. and Willerslev, E., 2007. Power and limitations of the chloroplast trnL (UAA) intron for plant DNA barcoding. In: Nucleic Acids Research, 35(3), e14. DOI: https://doi.org/10.1093/nar/gkl938
Taberlet, P.; Coissac, E.; Pompanon, F.; Brochmann, C. and Willerslev, E., 2012. Towards next-generation biodiversity assessment using DNA metabarcoding. In: Molecular Ecology, 21(8), pp. 2045-2050. DOI: https://doi.org/https://doi.org/10.1111/j.1365-294X.2012.05470.x
Tamura, K.; Nei, M. and Kumar, S., 2004. Prospects for inferring very large phylogenies by using the neighbor-joining method. In: Proceedings of the National Academy of Sciences, 101(30), pp. 11030-11035. DOI: https://doi.org/10.1073/pnas.0404206101
Tamura, K.; Stecher, G. and Kumar, S., 2021. MEGA11: Molecular Evolutionary Genetics Analysis version 11. In: Molecular Biology and Evolution, 38(7), pp.3022-3027. DOI: https://doi.org/10.1093/molbev/msab120
Tate, J.A. and Simpson, B.B., 2003. Paraphyly of Tarasa (Malvaceae) and diverse origins of the polyploid species. In: Systematic Botany, 28(4), pp. 723-737. DOI: https://doi.org/10.1043/02-64.1
Villanueva-Gutiérrez, R.; Moguel-Ordóñez, Y.; Echazarreta-González, C.M. and Arana-López, G., 2009. Monofloral honeys in the Yucatán Peninsula, Mexico. In: Grana, 48(3), pp. 214-223. DOI: https://doi.org/10.1080/00173130902929203


